NUCLEIC ACID-LIPID NANOPARTICLE AND METHOD USING THE SAME
US20250127882
2025-04-24
Human necessities
A61K39/215
Inventors:
Applicants:
Drawings (4 of 29)
Smart overview of the Invention
The invention introduces a nucleic acid-lipid nanoparticle designed to deliver nucleic acid molecules effectively. This nanoparticle combines a nucleic acid with a lipid mixture composed of ionizable amino lipids, phospholipids, cholesterol, and PEGylated lipids. The specific proportions of these components are critical for the nanoparticle's stability and functionality. The technology aims to enhance the delivery and efficacy of nucleic acid-based vaccines, particularly in the context of viral infections like COVID-19.
Background
Nucleic acid vaccines, especially those using mRNA, have gained attention due to their rapid development capabilities and effectiveness against viruses such as SARS-CoV-2. However, mRNA's inherent instability necessitates protection during delivery. Lipid nanoparticles (LNPs) have emerged as promising carriers that protect mRNA and facilitate its delivery into cells. Current mRNA vaccines require ultra-cold storage, limiting their distribution in areas without such facilities. In contrast, DNA vaccines are more stable but require additional methods for effective delivery and immune response induction.
Technical Composition
The nucleic acid-lipid nanoparticle described comprises a lipid mixture with precise molar percentages: 20-60% ionizable amino lipids for cellular uptake and endosomal escape, 5-20% phospholipids for structural support, 25-60% cholesterol for cell transfection, and 0.2-6% PEGylated lipids for stability and reduced protein binding. This composition ensures the nanoparticle's stability and enhances its ability to deliver nucleic acids effectively.
Applications
The invention outlines several methods utilizing the nucleic acid-lipid nanoparticle. These include preventing or treating coronavirus infections by administering the nanoparticle to induce an immune response. Additionally, it can be used as a booster dose for individuals previously vaccinated against coronavirus. This flexibility highlights the potential of these nanoparticles in various vaccination strategies.
Research and Development
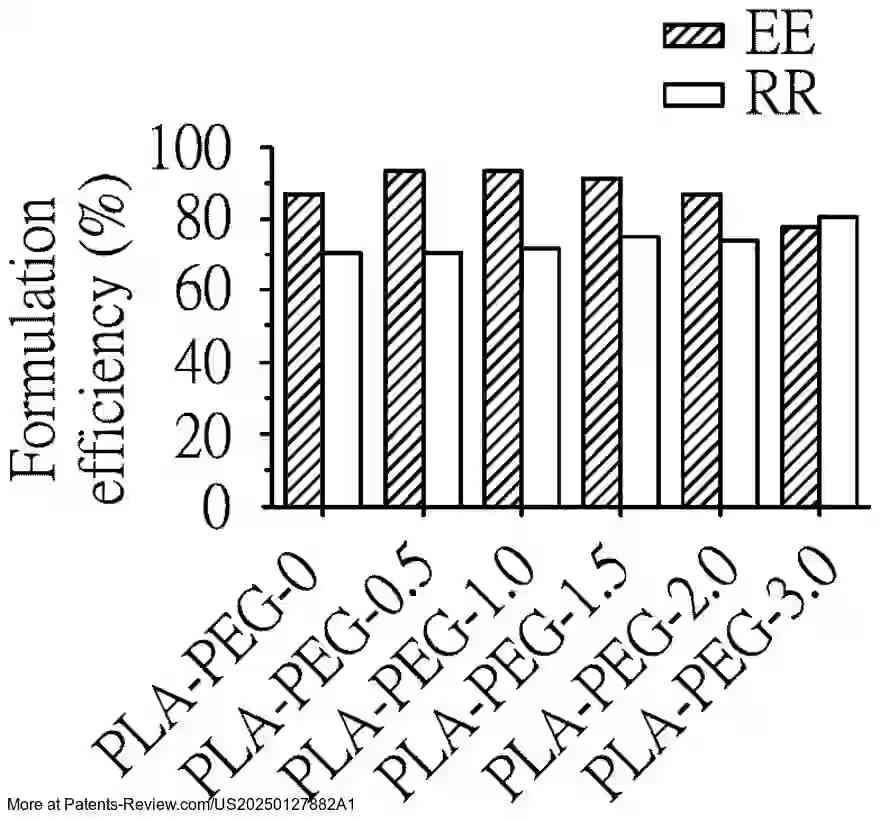
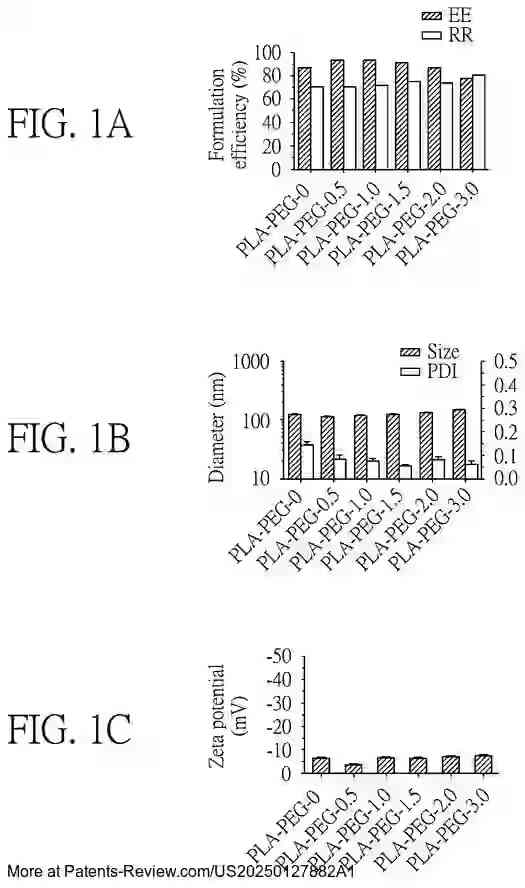
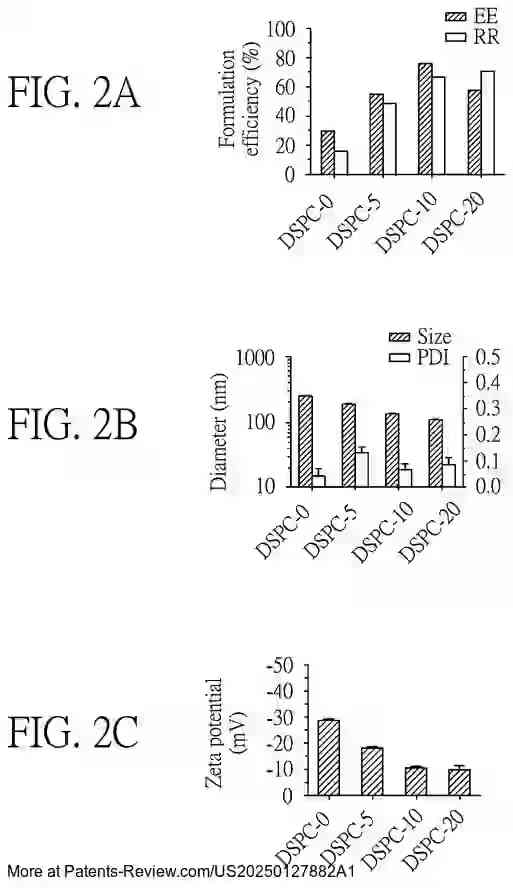
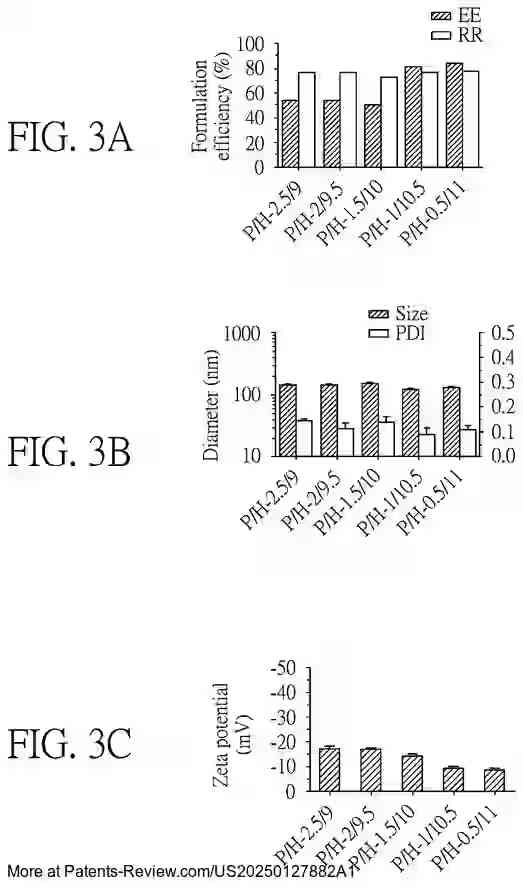
Extensive testing has been conducted on the formulation efficiency, particle size, and stability of these nanoparticles under different conditions. The accompanying diagrams illustrate these characteristics across various formulations and their performance in both in vitro and in vivo settings. The results demonstrate the promise of these nanoparticles in enhancing vaccine delivery and effectiveness, particularly against emerging variants like the SARS-CoV-2 Omicron variant.